Moderna is seeking Health Canada authorization for COVID-19 vaccine for kids as young as six months old.
Health Canada is reviewing a request for a COVID-19 vaccine for young children.
Moderna has applied for authorization of a COVID-19 shot for children between the ages of six months and five years old.
Earlier today, Health Canada updated its website, confirming the request has been received.
This comes one day after the company filed a similar request to the U.S. Food and Drug Administration, and the same day the Canadian government announced a new Moderna facility coming to Montreal to produce mRNA vaccines.
Last month, the company's vaccine for kids aged 6 to 11 was approved.
At the time, Moderna said trials indicate a low-dose of the shot works in babies, toddlers and preschoolers.


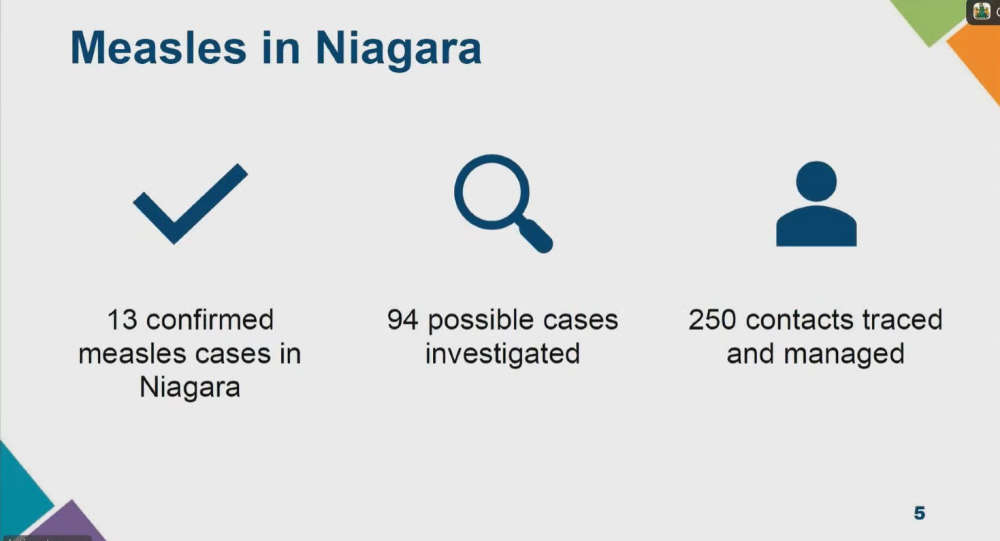 Region Updated on Measles Outbreak
Region Updated on Measles Outbreak
 St. Catharines Draws X Through X
St. Catharines Draws X Through X
 Police Looking for Laundry Thief
Police Looking for Laundry Thief
 Toronto Man Charged with Trafficking
Toronto Man Charged with Trafficking
 Niagara Falls Man Charged with Trafficking
Niagara Falls Man Charged with Trafficking
 Painting Honours Kristen French
Painting Honours Kristen French
 Keeping Douglas Memorial Public is Goal: Mayor
Keeping Douglas Memorial Public is Goal: Mayor
 Tourism Awards Received in Niagara
Tourism Awards Received in Niagara